Zika vaccines prove protective in mice; monkey study shows promise
CHICAGO – Reuters
AFP photo
Mice given a single shot of one of two experimental Zika vaccines were completely protected when exposed to the virus one to two months later, a promising sign that similar vaccines under development for humans will protect against Zika, U.S. researchers said on June 28.Separately, U.S. scientists said they have developed a model of the Zika virus in monkeys, a close proxy for human disease.
The studies advance efforts in fighting the mosquito-borne Zika virus, which has swept through the Americas and Caribbean since last fall, and has been linked to thousands of cases of microcephaly, a rare birth defect, in Brazil, as well as to neurological disorders. On Feb. 1, the World Health Organization declared Zika a global health emergency.
In the mouse study, published in the journal Nature, a team led by Dr. Dan Barouch of Beth Israel Deaconess Medical Center and Harvard Medical School, tested two different vaccine candidates in a strain of mice that develops Zika symptoms.
One candidate, a DNA vaccine, was developed by Barouch and colleagues. It contains bits of genetic material from a Zika virus strain from Brazil.
The other was made from a purified but inactivated version of the Zika virus from Puerto Rico. That vaccine was developed by researchers at Walter Reed Army Institute of Research in Silver Spring, Maryland.
Mice given either type of vaccine were 100 percent protected from Zika after a single shot. Unvaccinated mice that were exposed to the virus all developed symptoms of Zika.
Both types of vaccines - DNA and inactivated virus vaccines - have been successfully developed to prevent infection from viruses related to Zika, including West Nile and dengue.
The team also showed that antibodies taken from immunized mice could be used to protect other, unvaccinated mice, offering proof that the antibodies produced by the vaccines were specific to Zika.
“We need to be cautious about extrapolating data from a mouse model into humans,” Barouch said. But the fact that the vaccines protected mice and that their antibodies protected other mice from Zika was grounds for optimism over the development of a Zika vaccine, he said.
Study on monkeys
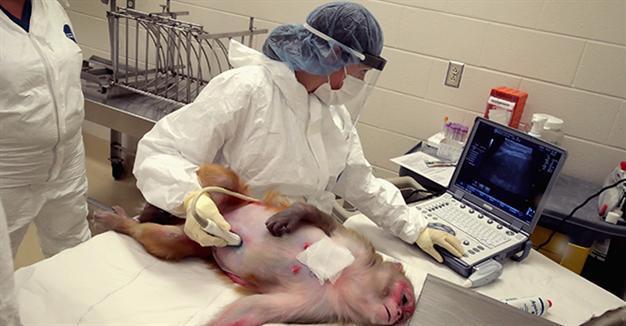
In another advance, researchers at the University of Wisconsin reported on June 28 that they have successfully infected rhesus macaques with an Asian strain of the Zika virus that is currently circulating in the Americas. The study, published in the journal Nature Communications, shows that monkeys - which have immune responses similar to humans - can be used to study Zika.
In the study, David O’Connor and colleagues inoculated eight rhesus macaques - including two pregnant monkeys - with a strain of Zika virus currently circulating in the Americas.
All eight animals were infected. Tests showed viral particles in their blood, saliva, urine and spinal fluid. All animals stayed infected for at least 21 days, and some remained infected for at least 57 days.
A second exposure to Zika 10 weeks after the first did not make the animals sick, suggesting that antibodies developed by the monkeys protected them against a second case of Zika. This is a promising sign that humans may similarly be able to develop protective antibodies against the virus.
The scientists are still working to understand whether the pregnant monkeys will pass the infection along to their fetuses, and whether infected monkeys can transmit the virus sexually, as has been seen in infected humans.
Meanwhile, U.S. lawmakers deadlocked over funding to fight the Zika virus on June 28, as Senate Democrats blocked a Republican proposal worth $1.1 billion they said fell short of the challenge posed by the mosquito-borne virus and hurt other health priorities.
















